Refrigerators, we use them every day and owe them, among other things, a greatly increased life expectancy even though very few can explain how they actually work. But we are about to change that.
Some would say that a refrigerator produces cold, but this is wrong from a physical point of view, since cold does not exist at all in physics. A distinction is only made between areas with higher energy, particle movement and temperature (outside the refrigerator) and lower particle motion, energy and temperature (inside the refrigerator). To put it simply, the refrigerator tends to transport heat from the food and its interior to the outside and leaves cold behind.
But to explain it more accurately we need to look at the two main physical concepts used in it:
The evaporation enthalpy. It describes the energy that a certain amount of liquid needs to change to the gaseous state. This energy can also be extracted from ambient heat, which is used to cool our fridges cooling system. For example, a deodorant bottle becomes very cold when sprayed for a long time.
The condensation/boiling point change of substances at pressure describes that substances have higher boiling points under high pressure (water famously boils at 100 degrees but this only apply at surface pressure at 5bar water boils at approx. 150 degrees).
Last but not least we need to look at something pretty obvious called heat exchange. We all know the pipes on the back wall of the refrigerator on the outside they are usually very dusty but hot and on the inside they are clean and cold. these pipes are responsible for heat exchange. The basic law of heat exchange states that when two bodies of different temperatures come into close contact with each other, the body of higher temperature gives off heat, the body of lower temperature absorbs heat. This is a fundamental concept in thermodynamics and physics.
This shows the main problem of the cooling cycle because the coolant inside the pipes in the fridge must be colder than the refrigerator interior itself. And on the other hand to release the heat on the pipes outside it must be warmer than the room temperature.
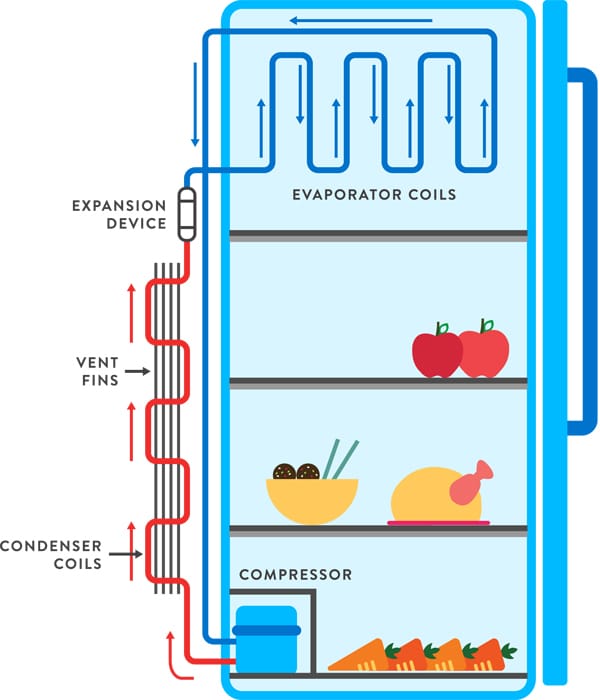
As stated above the fridge uses evaporation enthalpy to cool itself. A so-called expansion device is used for this purpose, which allows the coolant to evaporate through a nozzle by sudden pressure reduction into the evaporator coils, which accordingly cool down the liquid that has become gaseous and with it the inside of the refrigerator.
In order to maintain the low pressure inside the coils and to extract the already by the heat exchange heated gas, it is now fed into a compressor and put under strong pressure there. The important property of a coolant is to have a low boiling point (standard at 1bar approx. -12 degrees is the boiling point at which the liquid becomes gas). At this higher pressure, the gas is heated on the one hand, but at the same time the boiling point rises (standard for compressor 7 bar approx. 25 degrees boiling point) via the following condenser coils at the back of the refrigerator, the gas cools down by heat exchange with the outside air until it becomes liquid again (at the corresponding pressure at about 25 degrees) and it can now evaporate again through the expansion device and cool the refrigerator.
This way of explaining how it works shows beautifully how physicists and other scientists observe and experiment around to find said natural phenomena for then engineers to design devices and systems to put these phenomena to use and constantly improve them. And this is not just how our world worked back when refrigerators were invented but how it works to this day.
Thanks for reading, subscribe to the newsletter and here are some recommended sources to learn more:
